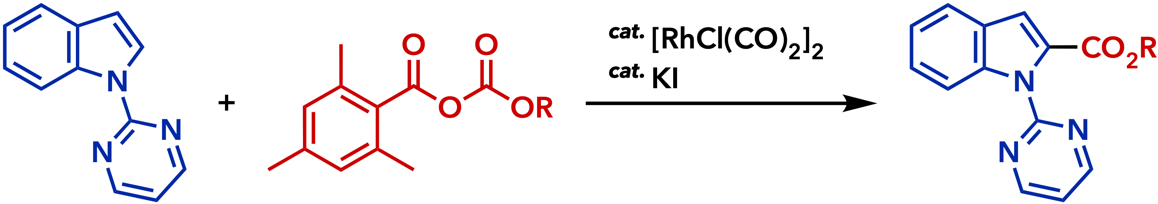
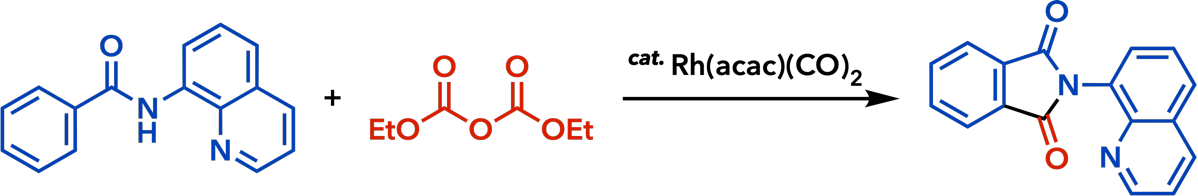
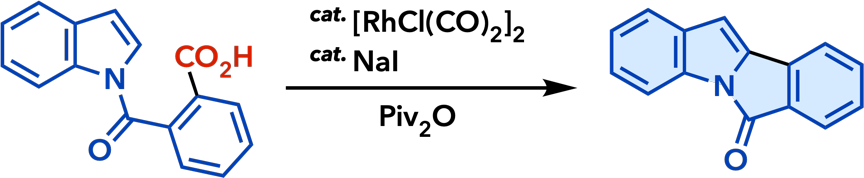
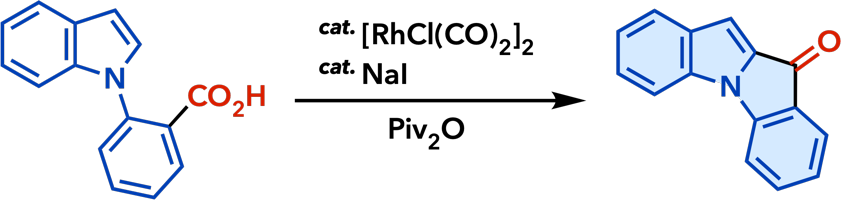
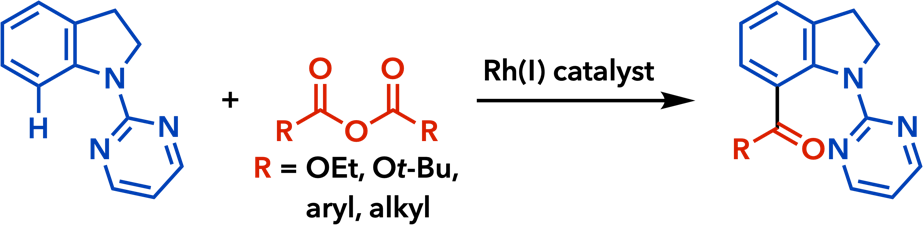
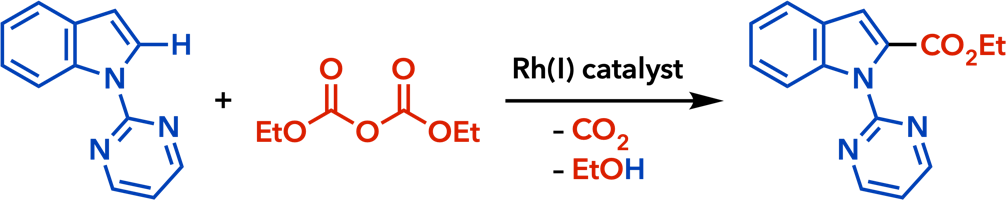
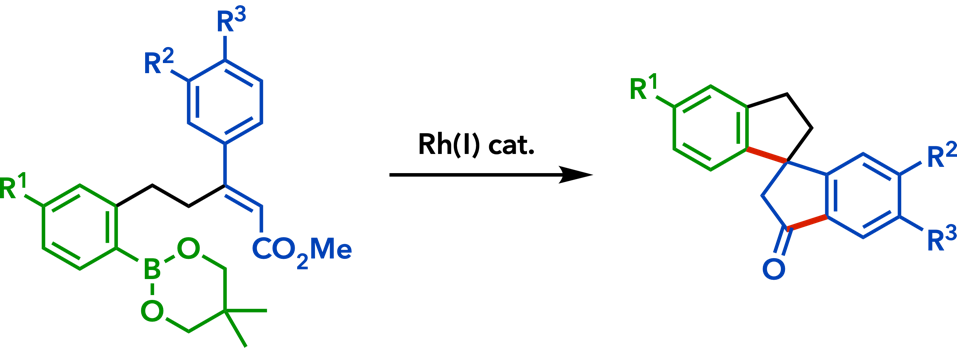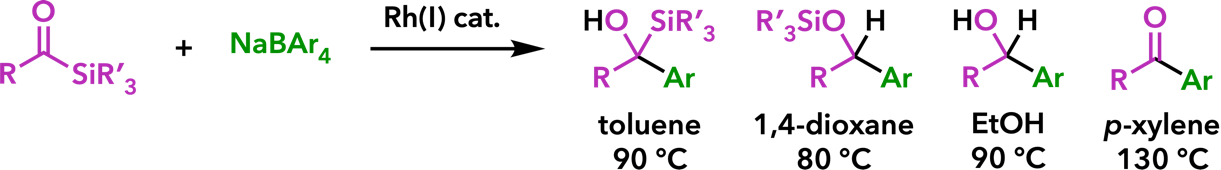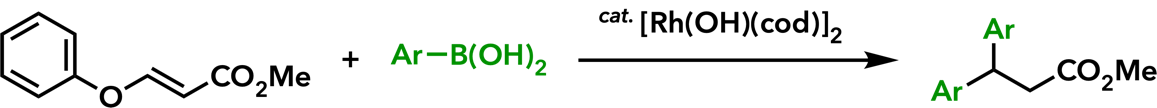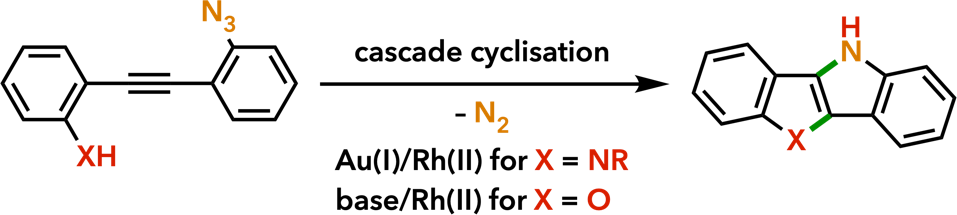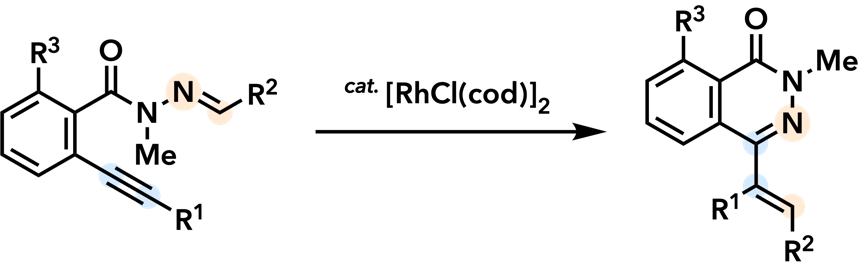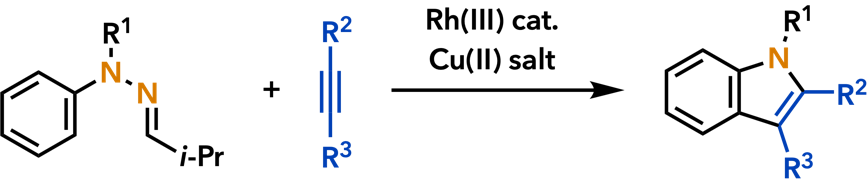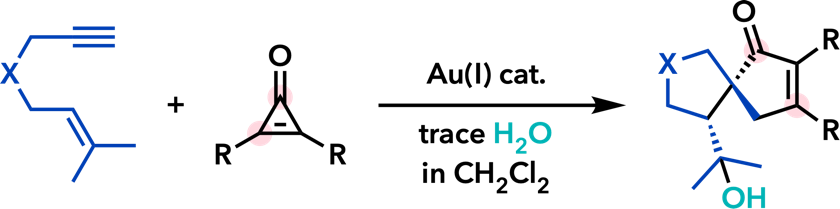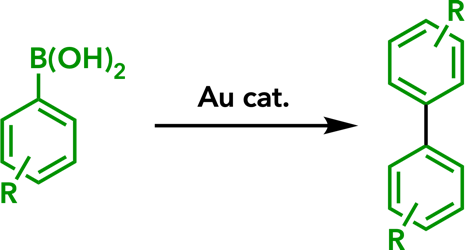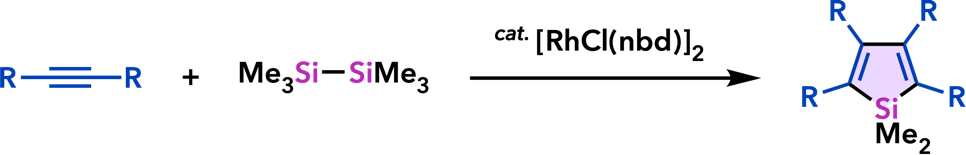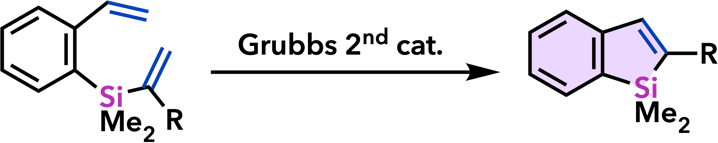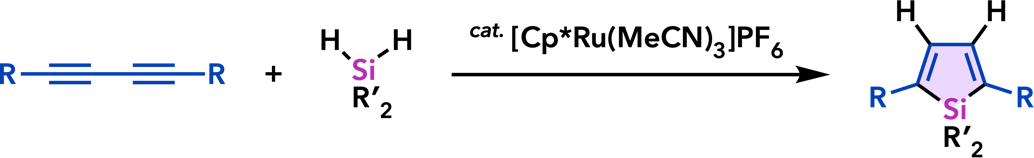2. Rhodium(I)-Catalyzed Addition Reactions of Arylboron Compounds |
|---|
| Eur. J. Org. Chem. 2020, 306. | 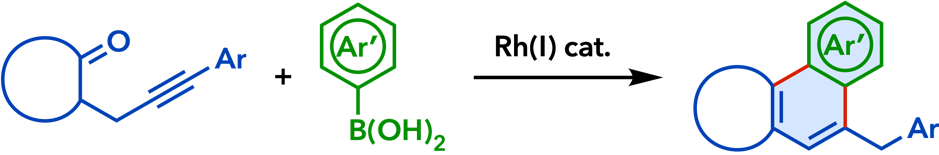 |
| Synlett 2015, 26, 1233. | 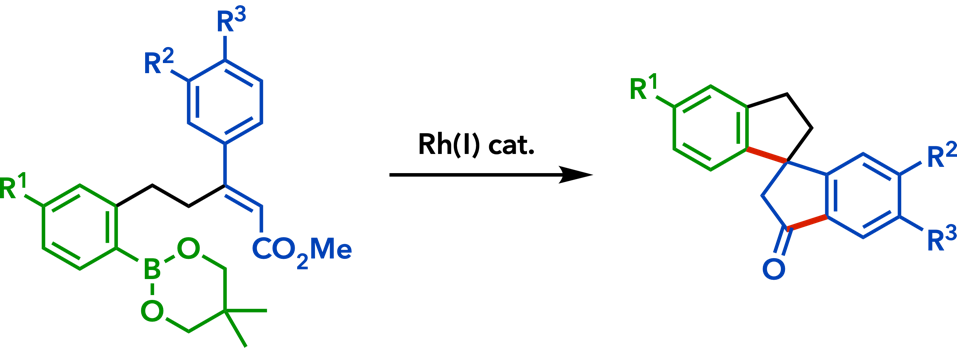 |
| Org. Biomol. Chem.� 2015, 13, 702. | 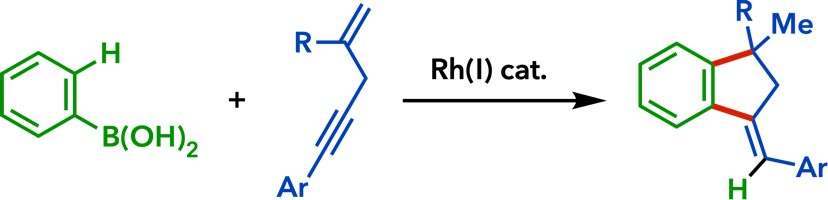 |
| J. Organomet. Chem. 2014, 765, 64. | 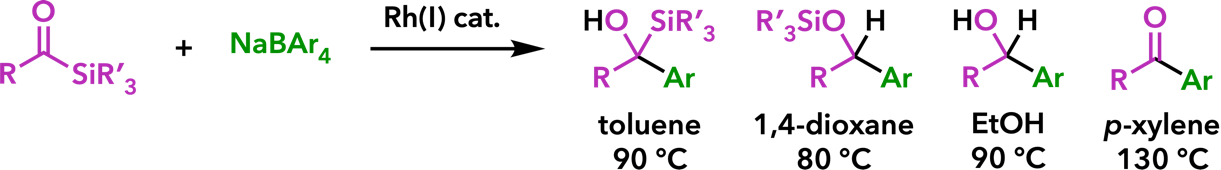 |
| Adv. Synth. Catal. 2013, 355, 3396. | 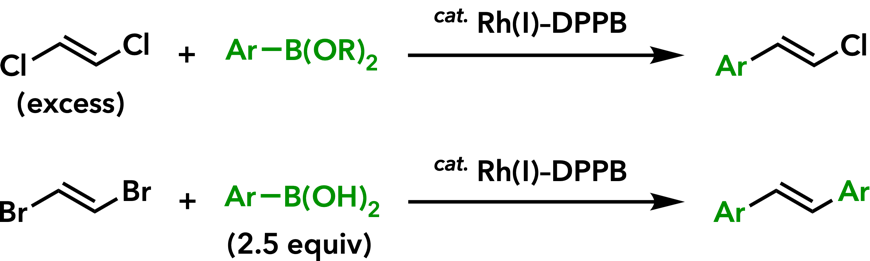 |
| Chem. Commun. 2012, 48, 2988. |  |
| Adv. Synth. Catal. 2011, 353, 1923. | 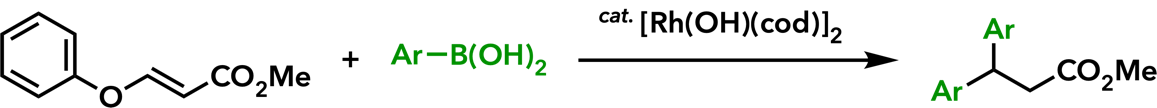 |
| Org. Lett. 2006, 8, 3379. | 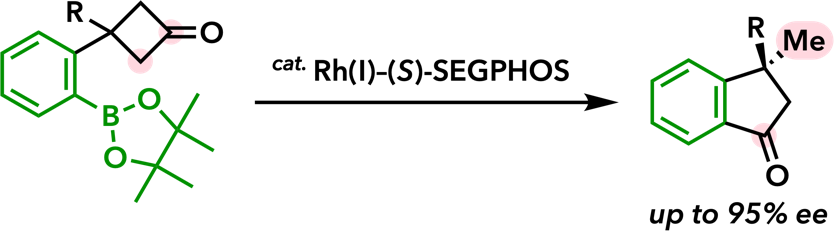 |
| Chem. Lett. 2005, 34, 1416. |  |
| Angew. Chem., Int. Ed. 2005, 44, 4608. | 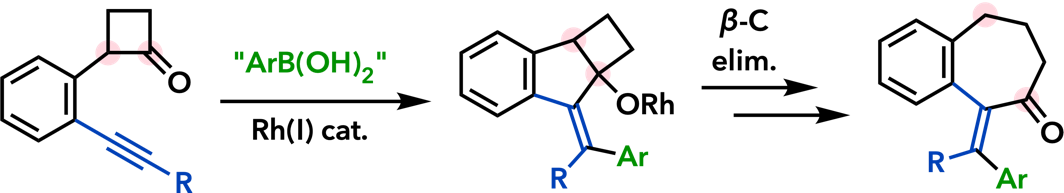 |
Bull. Chem. Soc. Jpn. 2005, 78, 1528.
Org. Lett. 2004, 6, 1257. |  |
3. Synthesis of Aromatic Compounds |
|---|
| Eur. J. Org. Chem. 2020, 306. |  |
| Org. Biomol. Chem. 2016, 14, 7024. | 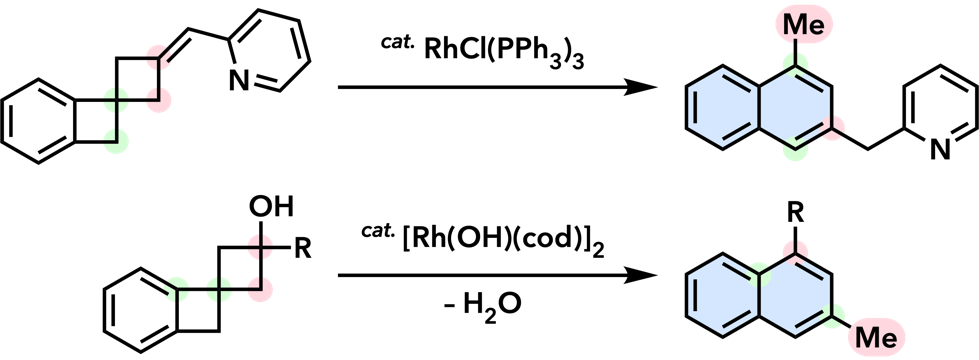 |
| Org. Biomol. Chem. 2016, 14, 5023. | 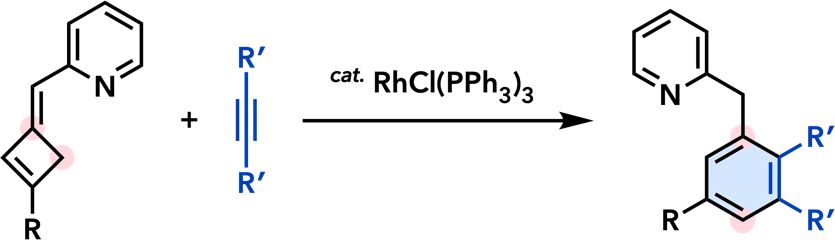 |
| Chem. Eur. J. 2016, 22, 1941. |  |
| Eur. J. Org. Chem. 2015, 3032. |  |
| Tetrahedron 2015, 71, 869. |  |
| Angew. Chem., Int. Ed. 2013, 52, 6492. |  |
| Org. Biomol. Chem. 2013, 11, 3424. | 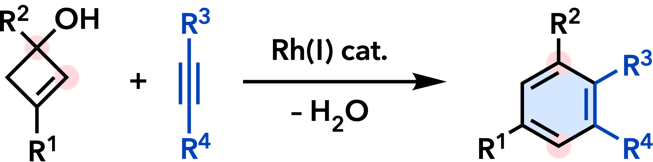 |
| Chem. Lett. 2011, 40, 40. | 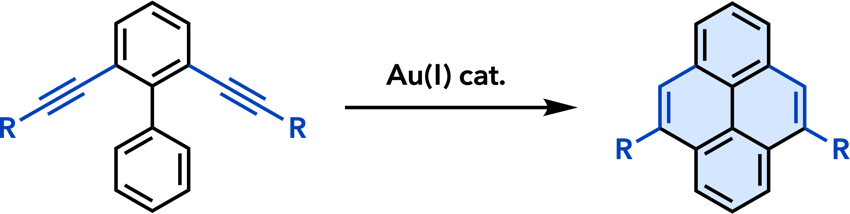 |
4. Successive C–C Bond Formation via 1,4-Rhodium Migration |
|---|
| Eur. J. Org. Chem. 2020, 306. |  |
| Synlett 2015, 26, 1233. | 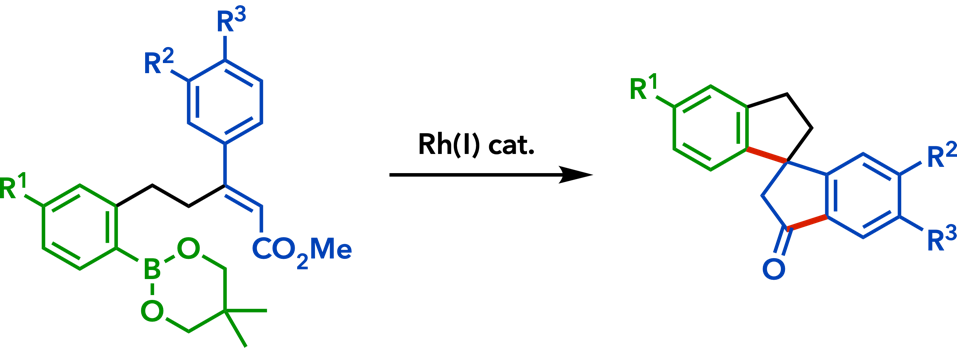 |
| Org. Biomol. Chem.� 2015, 13, 702. |  |
| Chem. Commun. 2012, 48, 2988. |  |
5. Ring Opening and Ring Expansion Reactions Involving β-Carbon Elimination |
|---|
| Synlett 2018, 29, 754. |  |
| Org. Biomol. Chem. 2016, 14, 7024. |  |
| Eur. J. Org. Chem. 2013, 4219. | 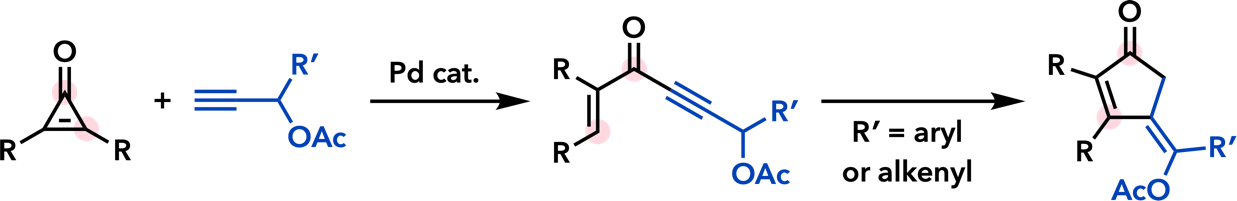 |
| Org. Biomol. Chem. 2013, 11, 3424. |  |
| Chem. Commun. 2012, 48, 2988. |  |
| Org. Lett. 2008, 10, 5219. | 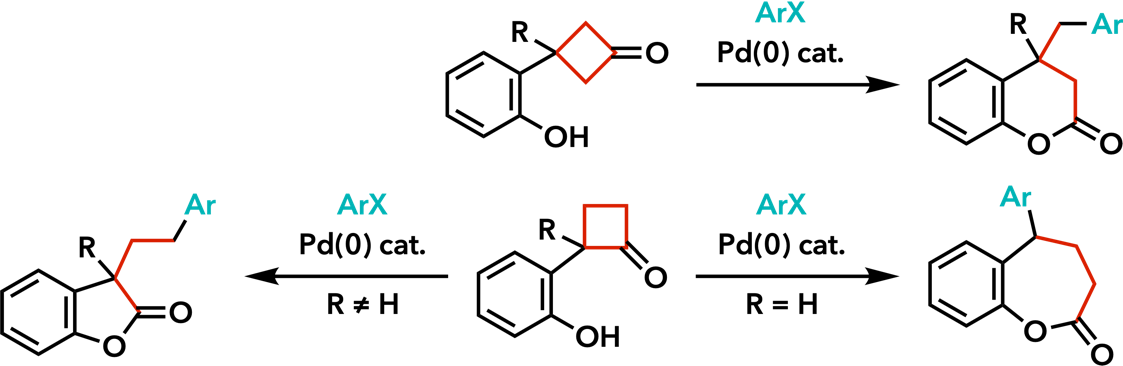 |
| J. Am. Chem. Soc. 2007, 129, 12596. |  |
| J. Am. Chem. Soc. 2007, 129, 12086. | 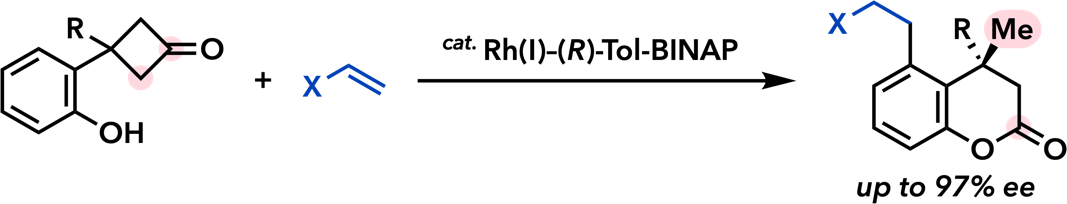 |
| Chem. Lett. 2007, 36, 744. | 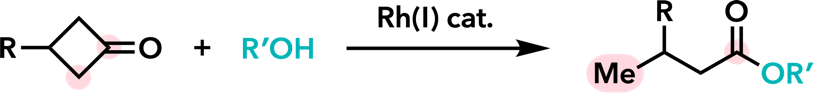 |
| Org. Lett. 2006, 8, 3379. |  |
| Angew. Chem., Int. Ed. 2005, 44, 4608. |  |
Bull. Chem. Soc. Jpn. 2005, 78, 1528.
Org. Lett. 2004, 6, 1257. |  |
6. Cyclization and Annulation Reactions Toward Nitrogen and Oxygen Heterocycles |
|---|
| Chimia 2018, 72, 888. |  |
| New J. Chem. 2018, 42, 19178. | 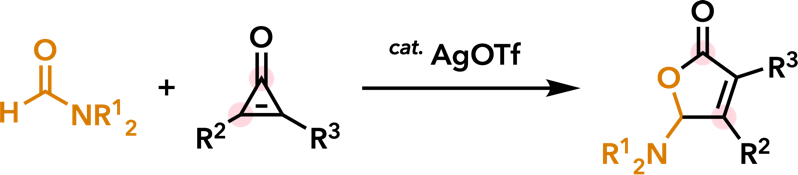 |
| Org. Biomol. Chem. 2018, 16, 6703. | 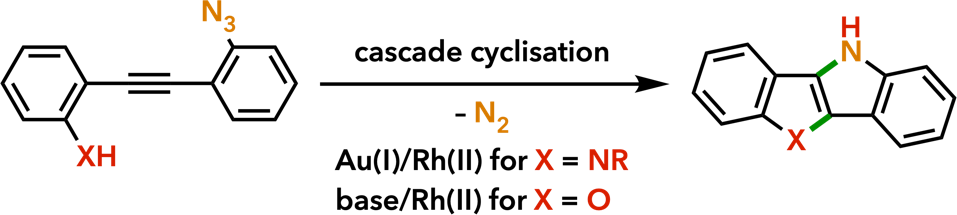 |
| Tetrahedron Lett. 2018, 59, 1458. | 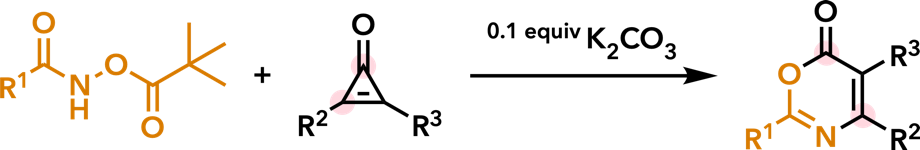 |
| Asian J. Org. Chem. 2016, 5, 891. | 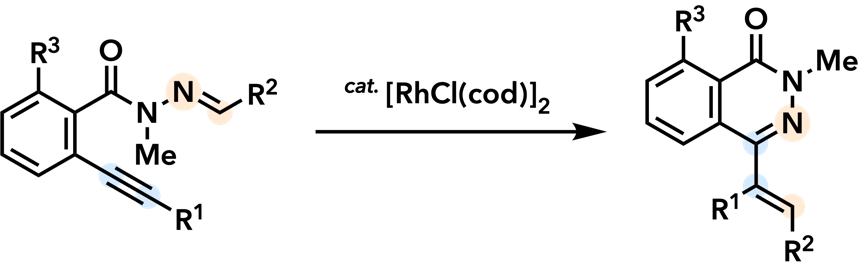 |
| Tetrahedron 2015, 71, 9264. | 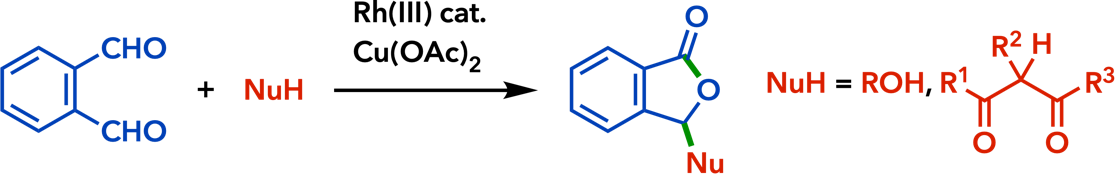 |
| RSC Adv. 2014, 4, 37138. | 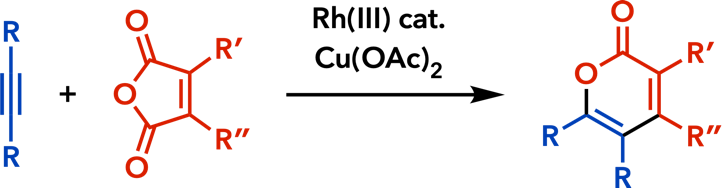 |
| Tetrahedron Lett. 2014, 55, 3302. | 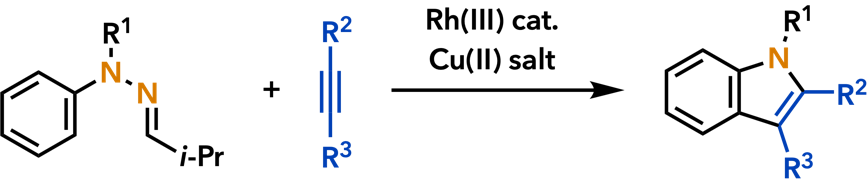 |
| Org. Biomol. Chem. 2013, 11, 2084. | 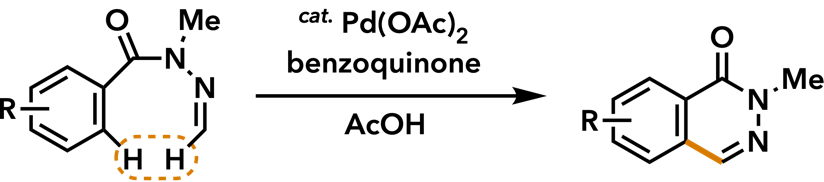 |
7. Gold- and Platinum-Catalyzed Reactions |
|---|
| Tetrahedron 2015, 71, 869. |  |
| J. Org. Chem. 2014, 79, 2739. | 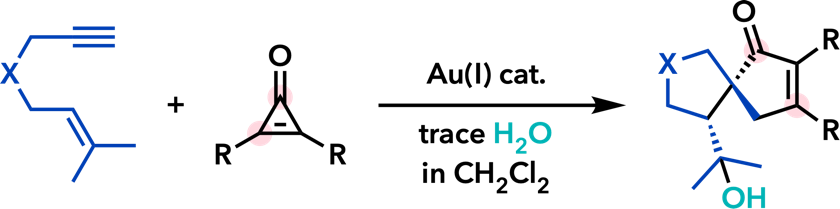 |
| Angew. Chem., Int. Ed. 2013, 52, 6492. |  |
| Tetrahedron Lett. 2011, 52, 4779. | 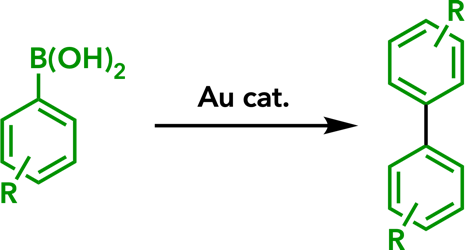 |
| Chem. Commun. 2011, 47, 8697. | 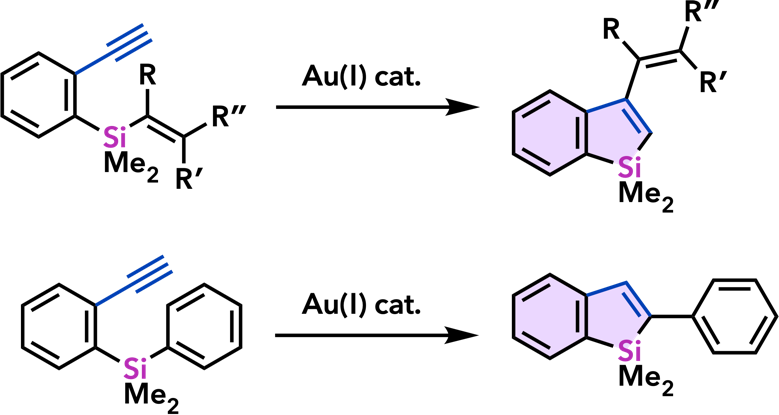 |
| Chem. Lett. 2011, 40, 40. |  |
| Chem. Commun. 2008, 2744. | 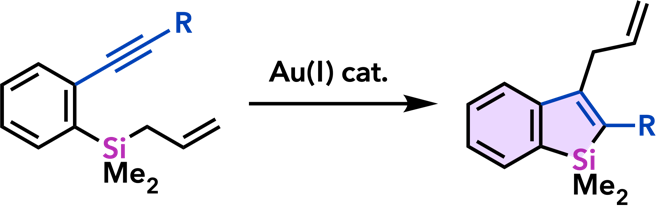 |
| Helv. Chim. Acta 2006, 89, 1672. |  |
| Synlett 2006, 575. | 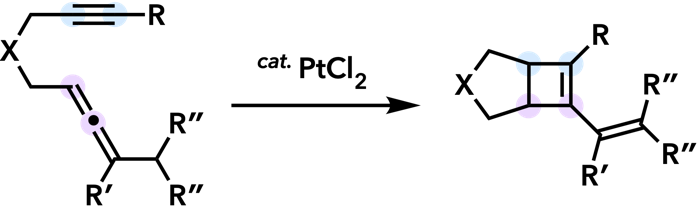 |
8. Rhodium(III)-Catalyzed Oxidative Transformations |
|---|
| Tetrahedron 2015, 71, 9264. |  |
| Eur. J. Org. Chem. 2015, 3032. |  |
| RSC Adv. 2014, 4, 37138. |  |
| Tetrahedron Lett. 2014, 55, 3302. | 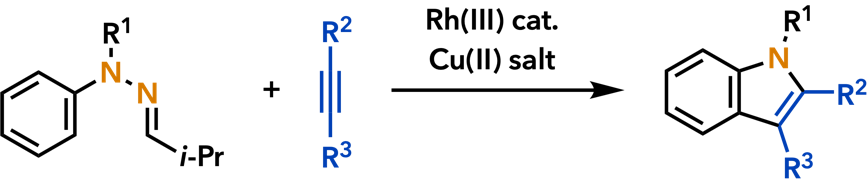 |
9. Catalytic Synthesis of Metalloles and Their Derivatives |
|---|
| Org. Biomol. Chem. 2012, 10, 3175. | 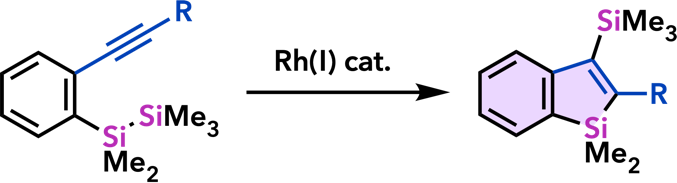 |
| Chem. Commun. 2011, 47, 8697. |  |
| Synlett 2011, 813. | 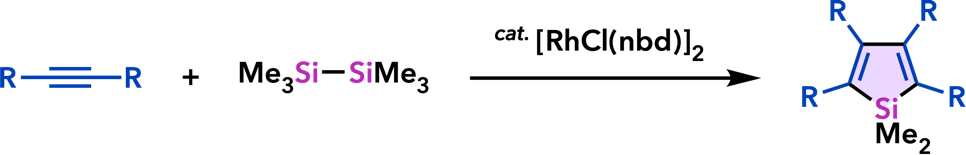 |
| Synlett 2010, 2743. |  |
| Org. Lett. 2010, 12, 1056. | 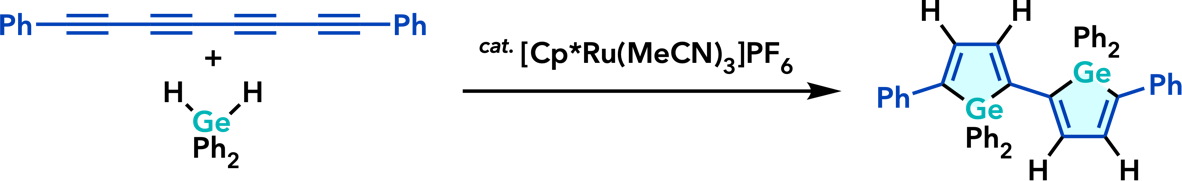 |
| Chem. Commun. 2008, 2744. |  |
| Synlett 2008, 561. | 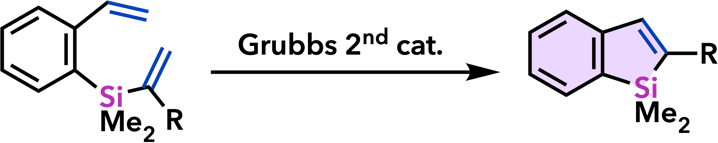 |
| Chem. Commun. 2007, 2627. | 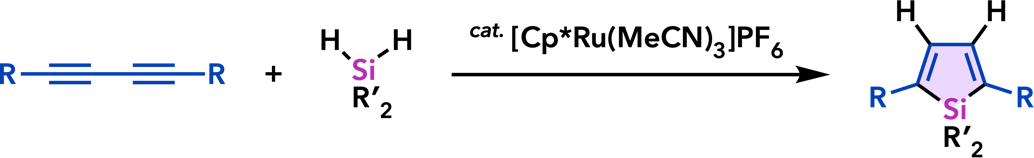 |
| Org. Lett. 2007, 9, 133. | 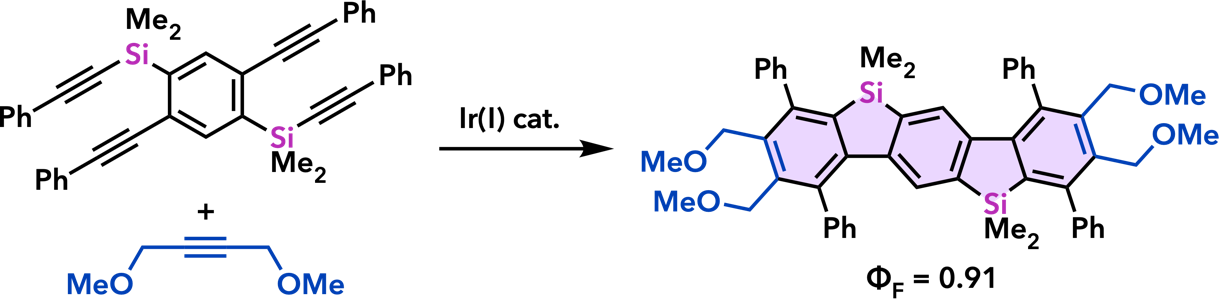 |