- Enterococcus hirae is resistant against high salt and high pH!
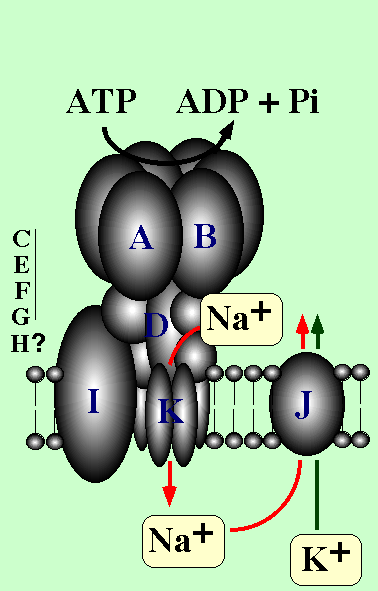 Enterococcus hirae is a gram-positive bacterium. It can grow
at high salt and alkaline pH condition. We found that sodium ion-pumping
ATPase in the cytoplasmic membrane is responsible for this property.
Furthermore, this ATPase is the first described V-type ATPase in an eubacterium.
Enterococcus hirae is a gram-positive bacterium. It can grow
at high salt and alkaline pH condition. We found that sodium ion-pumping
ATPase in the cytoplasmic membrane is responsible for this property.
Furthermore, this ATPase is the first described V-type ATPase in an eubacterium.
- What is V-type ATPase?
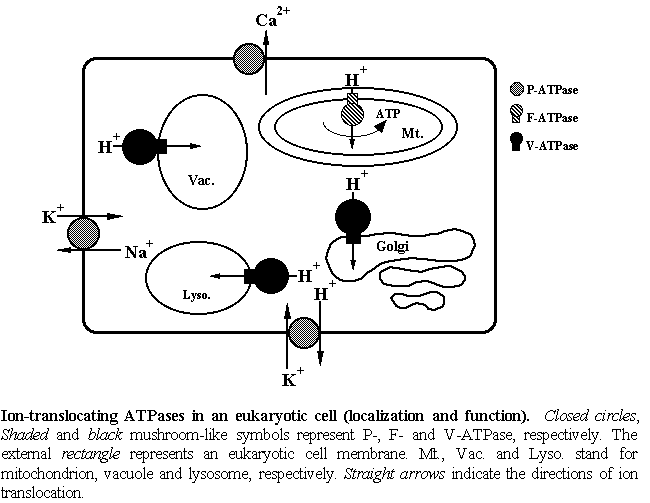 Homeostasis of ion concentrations in a cell is essential for all living cells,
including microorganisms, animals and plants. The homeostasis is maintained by
several ion transporting proteins, especially ion-pumping ATPases in cell membranes.
There are three types of ion-pumping ATPases, P-type ATPase in cytoplasmic membranes,
F-type ATPase in mitochondria, chloroplasts or cytoplasmic membranes of bacteria, and
V-type ATPase in organellar membranes. F-type and V-type ATPases are the relatives.
F-ATPase synthesizes ATP by using energy produced by oxidation of substrates in
mitochondria or by light absorption in chloroplasts. V-ATPase utilizes ATP to pump
out ions, usually protons in organella in eukaryotic cells.
Homeostasis of ion concentrations in a cell is essential for all living cells,
including microorganisms, animals and plants. The homeostasis is maintained by
several ion transporting proteins, especially ion-pumping ATPases in cell membranes.
There are three types of ion-pumping ATPases, P-type ATPase in cytoplasmic membranes,
F-type ATPase in mitochondria, chloroplasts or cytoplasmic membranes of bacteria, and
V-type ATPase in organellar membranes. F-type and V-type ATPases are the relatives.
F-ATPase synthesizes ATP by using energy produced by oxidation of substrates in
mitochondria or by light absorption in chloroplasts. V-ATPase utilizes ATP to pump
out ions, usually protons in organella in eukaryotic cells.V-ATPase is important in the pH maintenance (acidification) of inside of organella or outside of osteoclasts. The acidification is profoundly related to cholesterol metabolism, storage of metabolites, autophagy, etc in organella and also to osteoclasis. And these functions are related to many diseases, such as arterial sclerosis, osteoporosis and so on. The acidification mechanism and its regulation, especially those of V-ATPase are studied by many researchers.
We found a bacterial V-ATPase. Bacterial V-type ATPase is produced in a large amount and
easily purified. Therefore, we expect that the biochemical and molecular biological studies
of our ATPase should give us a fundamental understanding of the properties of V-type ATPases.
 What we have done
What we have done What we are going to do
What we are going to do Back to "Study" Home Page
Back to "Study" Home Page Yamato Lab. Home Page
Yamato Lab. Home Page