LABORATORY

Tokyo University of Science,
Laboratory of Pharmaceutical Quality Design and GMP
Professor Shingou Sakurai
Introduction of the Laboratory
In July 2020, I was appointed as a professor of the social collaboration course “Pharmaceutical Quality and GMP Course” newly established at our university. The responsible persons of this course are Professor Mayumi Shikano, Director of The Support Center for Clinical Pharmacy Education, who supervises the regulatory science training course, and Chairman Motoharu Kinoshita, the Chemo-Sero-Therapeutic Research Institute which is the collaborator of the course. Prof. Shikano was a former reviewer for PMDA and has been a professor at our university since April 2018. She has been concerned about few opportunities for education on pharmaceutical quality and GMP in the pharmacology. On the other hand, the Chemo-Sero-Therapeutic Research Institute is a foundation which promotes public benefit projects such as subsidies for biologics research including fundamental fields, scholarships for promising human resources and awards, and has recognized the necessity of GMP education in academia. Such awareness of the issues has brought the collaboration, and this course, which I am responsible for, was established in July 2020. Since then, the course is being operated with support from Japan Generic Medicines Association, Toyama Household Medicine Makers Association and several other companies in addition to the Chemo-Sero-Therapeutic Research Institute.
The first course in the academia aims to contribute to the society with the following three objectives by supporting enhancement of the industry-government-academia collaboration through education and research activities on pharmaceutical quality and GMP:
- Distribution of high-quality pharmaceuticals,
- Efficient application of innovative production technologies, and
- Proper and efficient operation of quality-related regulations.
The ultimate goal of the course is to build an all-Japan system in the GMP field, and gain more collaborators to promote the penetration of GMP as well as to improve knowledge and understanding of GMP among domestic manufacturers and government officials.
As many of you know, GMP violations and misconduct by generic drug companies occurred in 2019, which have led to an instability in the supply of pharmaceuticals. Furthermore, we are facing delays of domestic development and production of COVID-19 vaccines. Based on this experience, several national projects are moving toward domestic production of vaccines and other products in order to deal with possible emerging and re-emerging infectious diseases in the future. GMP human resource development is directly linked to solving these problems. In addition, Pharmaceutical Affairs Law was revised in 2004, which made it possible to outsource the entire manufacturing of pharmaceuticals. It caused the progress of split-off of a company to separate marketing operation from manufacturing operation, which is bringing the significant trend toward a shortage of GMP human resources to marketing authorization holders. The development of GMP human resources at pharmaceutical companies, which support the Japanese pharmaceutical industry, is just desired now.
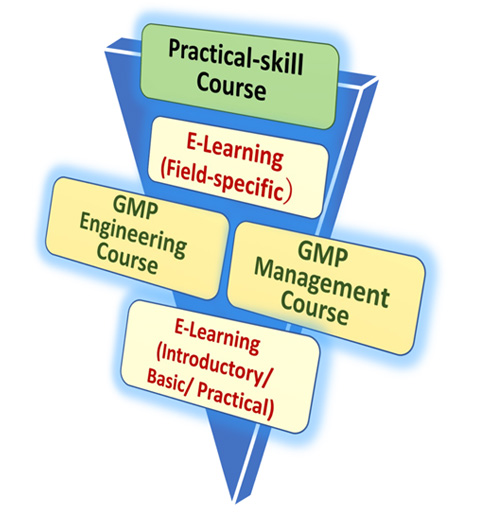
In our laboratory which was established at this timing, we are now preparing GMP Education and Training Course using e-learning and HyFlex (Hybrid/flexible) systems (Fig. 1) so that working adults as well as students can receive the education. Already, in October 2021, E-learning was introduced, and the delivery of the basic course began. In April 2022, the practical course was also distributed. Specialized courses broadcasted in October 2022. Furthermore, in the same month, we introduced the first GMP-compliant engineering course, and in April 2023, we commenced the GMP-compliant management course. We will organize a program so that security pharmacists and those who are engaged in quality assurance departments (QA) and GQP departments of marketing authorization holders, who are the cornerstones of GMP at manufacturing sites, will become experts in GMP in about 1 to 2 years. These programs refer to several Qualified Person (QP) training courses used in the QP system, which is a reasonable framework in the EU, and is considered in light of Japanese regulatory requirements and specific matters. We believe that this is a supportive system in line with the PIC/S Authorized Person (AP) system (same as the EU QP system). Security Pharmacists in Japan must be qualified as pharmacists, but GMP is not as firmly incorporated into pharmaceutical education as it is in Europe and the United States. In these circumstances, we believe that the GMP education and training course currently being set up will be a distinctive educational program at our university. We have already received a lot of support and cooperation on the GMP-compliant engineering and GMP-compliant management courses.
In addition to establishment of the above education and training courses, our laboratory uses public research funds to conduct a research on international harmonization of guidelines for manufacturing control and quality control of pharmaceuticals, regenerative medicine products, and medical devices ("Research on international harmonization of guidelines for GMP, QMS, and GCTP"; principal researcher: Shingo Sakurai), a research on quality control methods for regenerative medicine products (cell processed products) ("Establishment of QbD-based control strategy and Advanced Core Ecosystem in cell manufacturing"; principal researcher: Professor Masahiro Kino-oka (Osaka University)), and a research on creating educational materials for human resource development for new modalities (mRNA vaccines, etc.) (”Creation of an educational program that contributes to the development of human resources involved in advanced production technology for vaccines and other products with new bio-manufacturing methods”; principal researcher: Dr. Satoshi Toyoshima (Representative Director of Biologicals Center for Research and Training; BCRET)).
In the midst of remarkable progress in science and technology, we set the laboratory’s task as research on how to proceed with the quality assurance of pharmaceuticals and regenerative medicine products including new modalities, harmonization of regulations (regulatory science) through research on global standards and guidelines, and connecting the latest findings obtained from the above research to human resource development. It would be our greatest pleasure if our research ultimately resulted in the safety and security of patients.
Activity Overview
GMP Training at Manufacturing Sites
-
E-learning
To make GMP easy to understand for employees at all levels of manufacturing plants, programs are developed in collaboration with publishers, and being delivered sequentially. Educational materials are divided into the GMP common area and GMP field-specific area. In the common area, we have established an introductory course, basic course, and practical course, which are designed so that people of all levels, from beginners to managers, can receive appropriate education and training from all perspectives of regulation and technology.
Contribution to New Production Technology
-
Fundamental development project for manufacturing regenerative medicine products based on QbD
A research activity of the Japan Agency for Medical Research and Development (AMED) are being conducted with the theme of "Establishment of QbD-based control strategy and Advanced Core Ecosystem in cell manufacturing ". Under a working group (WG) operated by Professor Masahiro Kino-oka (Department of Biotechnology, Graduate School of Engineering, Osaka University), our laboratory is acting as the regulation and system WG, and investigating the status of overseas regulations and how the QbD approach for regenerative medicine products should be handled in terms of regulations.
-
Creation of an educational program that contributes to the development of human resources involved in advanced production technology for vaccines and other products with new bio-manufacturing methods
As an AMED research activity, we are developing educational materials with Dr. Satoshi Toyoshima (Biologicals Center for Research and Training; BCRET) as the research and development representative, for those involved in the manufacture and development of new modality vaccines using mRNA and viral vectors seen in recent novel coronavirus vaccines. Among them, Sakurai team is making the materials on the industrial production (regulatory requirements, manufacturing control and quality control).
Health, Labor and Welfare Administration Promotion Survey Project, “Research on international harmonization of guidelines for GMP, QMS, and GCTP”
-
GMP research group activities
We drafted the GMP Ordinance that came into effect in 2021. In addition, we are examining draft audit manuals to contribute to the effective operation of pharmaceutical quality systems and effective audits of manufacturing sites by marketing authorization holders.
-
QMS research group activities
We have drafted a QMS ministerial ordinance revision and created clause-by-clause explanation for medical devices and in-vitro diagnostics.
-
GCTP research group activities
We have prepared guidelines for aseptic manufacturing methods for regenerative medicine products and related FAQs. We are also examining the GCTP draft for investigational products and the revision of the GCTP ministerial ordinance.
Cooperation with other institutions
We are conducting joint research activities in collaboration with Kumamoto Health Science University, Sanyo-Onoda Municipal Yamaguchi Tokyo University of Science, and the Faculty of Pharmaceutical Sciences, University of Toyama, which have GMP-related courses. In addition, we have established a research cooperation system based on a joint research memorandum with the Pharmaceuticals and Medical Devices Agency (PMDA), and we are conducting research on the theme of innovative manufacturing technology, in addition to reviewing revisions of GMP ordinances and GCTP ordinances. On the other hand, with regard to industry, we have received research grants from the Chemo-Sero-Therapeutic Research Institute and other organizations, and the knowledge on manufacturing and quality-related operations, etc. from industry groups such as the Federation of Pharmaceutical Manufacturers' Associations of Japan to continue our research activities.
