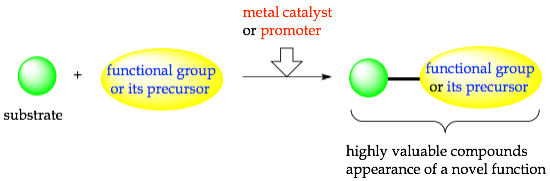
Introduction of a Functional Group
Introduction of a specific functional group onto a substrate with a metal catalyst or a promoter led to the production of highly valuable compounds, an appearance of a novel function, or the unprecedented reaction
1) Trimethylchlorosilane (TMSCl) promoted the coupling reaction of a Reformatsky reagent with N,O-acetals, which functioned as an amino acid precursor, producing the α-amino acid derivatives.
2) The use of hafnium triflate [Hf(OTf)4], a hard Lewis acid, regioselectively catalyzed N-aminomethylation on the nitrogen atom of indoles to produce the kinetically stable products.
1) The CoCl2-TBHP oxidizing system efficiently catalyzed cyanation of a C-H bond on tertiary aromatic amines.
Eur. J. Org. Chem. 2009, 917.
J. Org. Chem. 2010, 75, 3923.
トリメチルクロロシラン(Me3SiCl)が、N,O-アセタールを用いたレフォルマトスキー(Reformatsky)型反応を促進することを見出し、アミノ酸誘導体の全く新しい骨格合成法を開発しました。
非天然型アミノ酸
N,O-アセタール
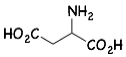

アスパラギン酸

Introduction of Specific Functional Groups

Copyright @ Sakai group in TUS All Rights Reserved
produced by Mac

Synlett 2013, 24, 1283.
2) The Cu(II)-DBU system successfully catalyzed an oxidative N-nitrosation of secondary and tertiary amines with nitromethane as a nitroso source under oxygen atmosphere.
Chem. Commun. 2015, 51, 11638.
1) Introduction of a functional group through a typical substitution
2) Oxidative introduction of a functional group
(conventional introduction)
novel introduction

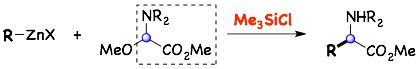
3) Application to a cross-coupling with a substrate having a non-activated bond
1) A palladium-phosphine catalytic system efficiently promoted acyl-aryl Hiyama coupling of acyl halides with arylsilanes in the presence of CsF, producing diaryl ketone derivatives. This acyl fluorides could be applied to a cross-coupling partner in Suzuki-Miyaura coupling.
Chem. Lett. 2016, 45, 790.
Eur. J. Org. Chem. 2017, 4324.
パラジウム/リン配位子の組み合わせが、ハロゲン化アシルと有機ケイ素反応剤のHIyama型クロスカップリング反応を効率よく促進しジアリールケトン類を合成することを新たに見出しました。
また、フッ化アシルは、ボロン酸とのSuzuki-Miyaura型クロスカップリング反応の基質に適用すできることも新たに見出しました。


インドールへのN,O-アセタールの導入は、これまで3位炭素上で起こるのが一般的でしたが、ハード(固い)ルイス酸であるハフニウムトリフラートを用いると、1位窒素上での導入が、位置選択的に起こることを新たに見出しました。
>>




3) The Cu(II)-DMAP-base system efficiently catalyzed cyanation of aryl iodides leading to the preparation of benzonitrile derivatives. It was also found that in this system nitromethane functions as a cyano source.
Chem. Lett. 2017, 46, 1736.