Research
Introduction
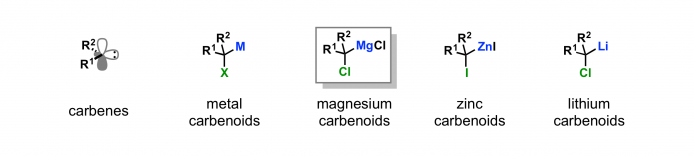
Organometallic compounds possessing a good leaving group at the α-position are referred to as metal carbenoids. The [2+1] cycloaddition of zinc carbenoids with alkenes is known as the Simmons–Smith reaction. The chemistry of lithium carbenoids has long been studied in great depth: however the lithium carbenoids are unstable and not so practical. Magnesium carbenoids are organomagnesium compounds possessing a leaving group (typically a halo group) at the α-position.
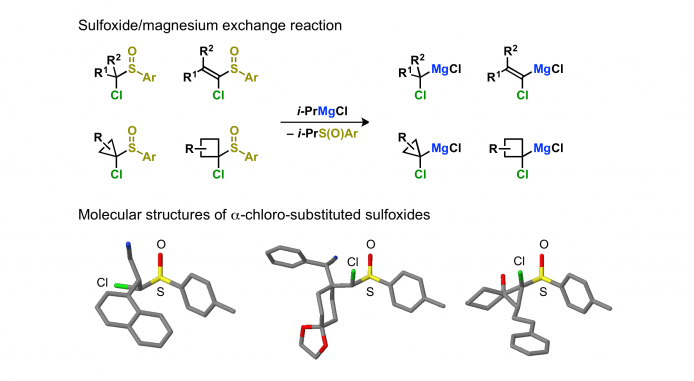
Magnesium carbenoids can be generated from α-chloro-substituted sulfoxides and Grignard reagents via the sulfoxide/magnesium exchange reaction. In most cases, the resulting magnesium carbenoids are stable at –78 °C for 30 min. The exchange reaction is chemoselective, and magnesium carbenoids possessing an electrophilic functional group can be generated via the sulfoxide/magnesium exchange reaction. S-Chiral p-tolylsulfinyl group can be used as a chiral auxiliary.
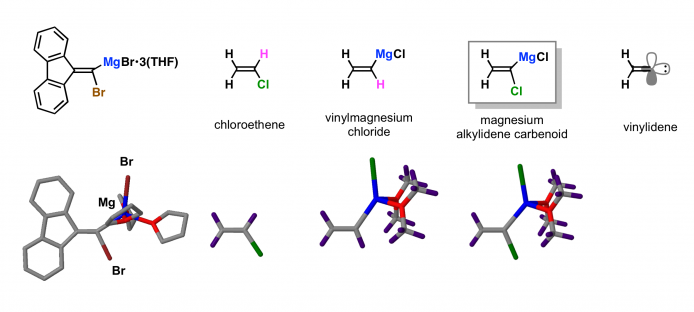
The reactivity of magnesium carbenoids is similar to that of carbenes. In addition, the magnesium carbenoids show ambiphilic reactivity, that is, the magnesium carbenoids react not only with electrophiles but also nucleophiles. The nucleophilic substitution occurs at the vinylic carbon atom of magnesium alkylidene carbenoids.

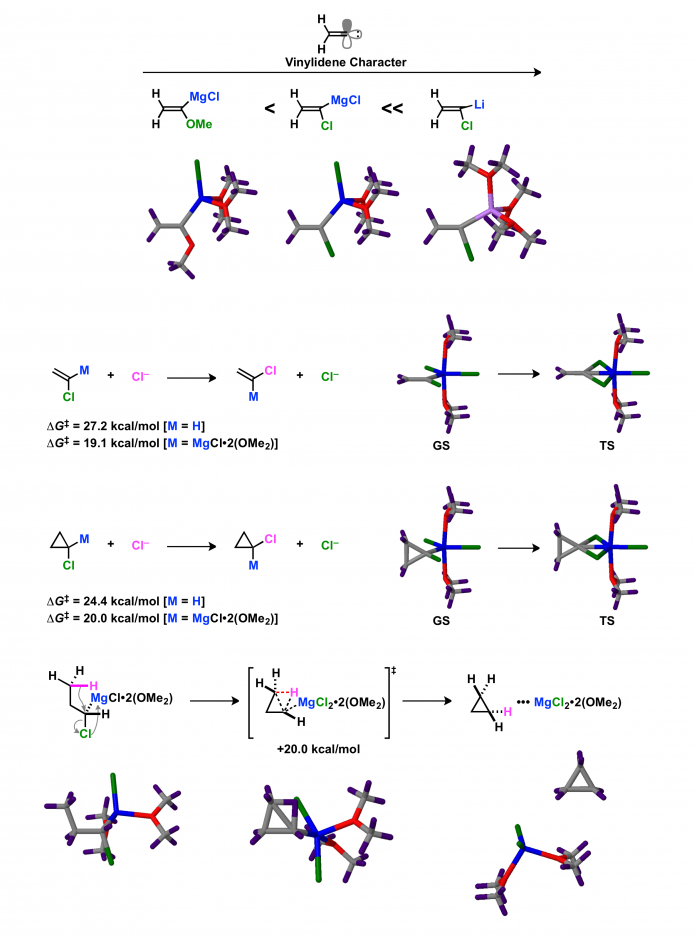
The structure of magnesium alkylidene carbenoid has been determined by single-crystal X-ray diffraction structural analysis (Boche, G.; Harms, K.; Marsch, M.; Müller, A. J. Chem. Soc., Chem. Commun. 1994, 1393 http://pubs.rsc.org/en/Content/ArticleLanding/1994/C3/C39940001393#!divAbstract). The structure of magnesium alkylidene carbenoid deviates from the standard organic molecules. In the DFT study, the structure of magnesium alkylidene carbenoid is characterized by long C–X bond, large C=C–Mg bond angle, and small C=C–Cl bond angle. The geometry of magnesium alkylidene carbenoid is halfway between trigonal chloroethene and linear vinylidene.
Resent Research Themes
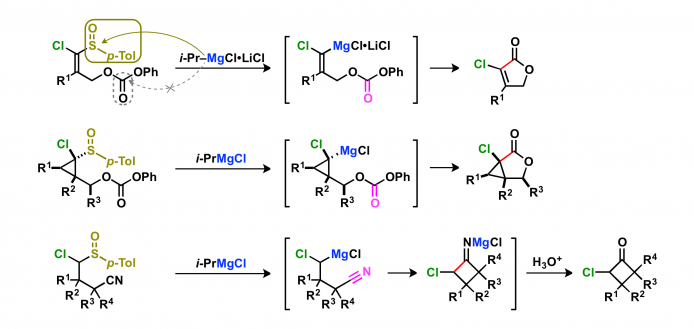
1. Nucleophilic reactions of magnesium carbenoids
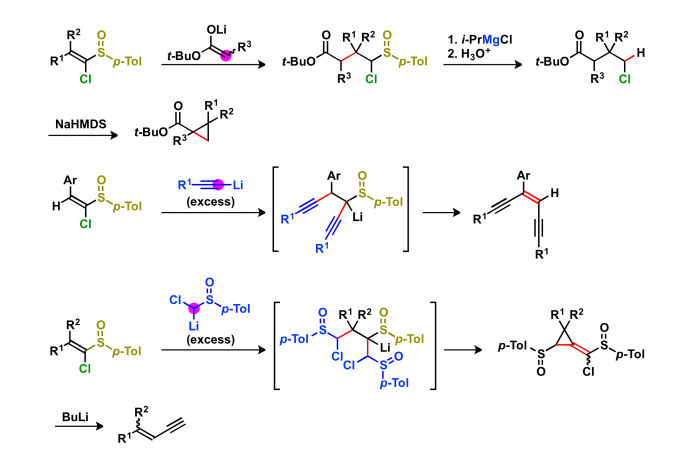
Magnesium carbenoids act as nucleophiles in a similar manner to that of Grignard reagents. When the magnesium carbenoids are generated from α-chloro-substituted sulfoxides possessing an electrophilic functional group, the intramolecular reactions occur to give cyclic compounds.
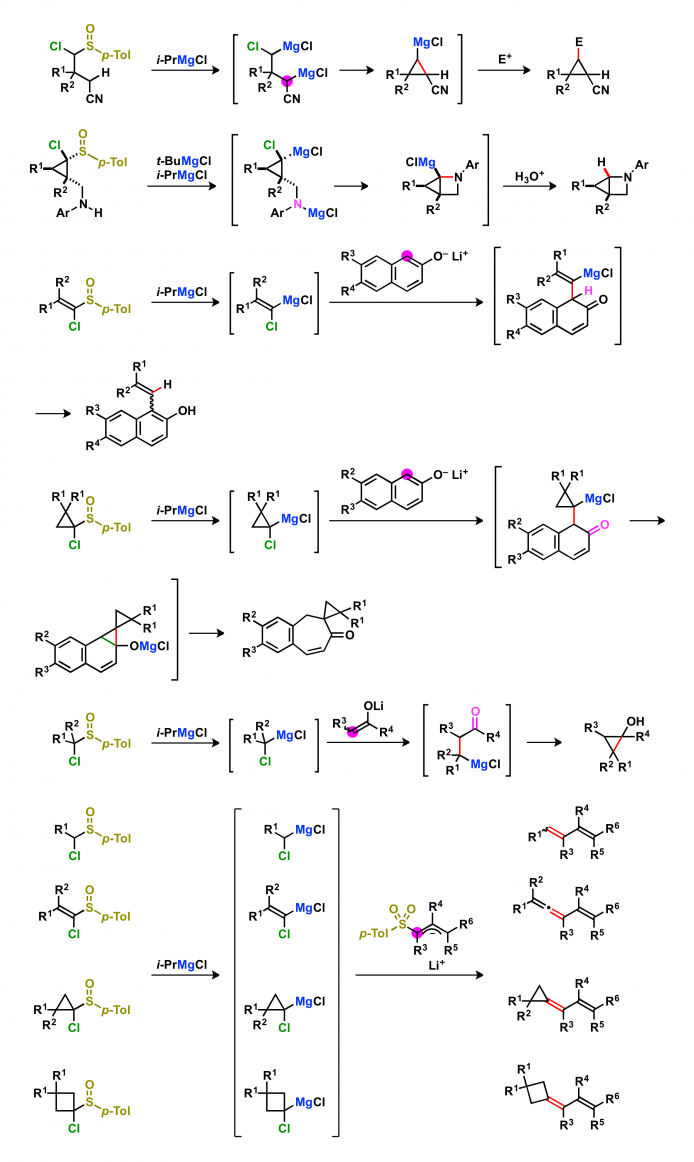
2. Electrophilic reactions of magnesium carbenoids
Magnesium carbenoids act as electrophiles in a similar manner to that of organic chlorides. The resulting organomagnesium intermediates can be converted into a variety of compounds. The reactions of organomagnesium intermediates with electrophiles give three-component coupling products in one-pot. When the magnesium carbenoids are reacted with nucleophiles possessing an electrophilic functional group, the annulations occur to give cyclic compounds. The reactions of magnesium carbenoids with nucleophiles possessing a leaving group afford alkenes.
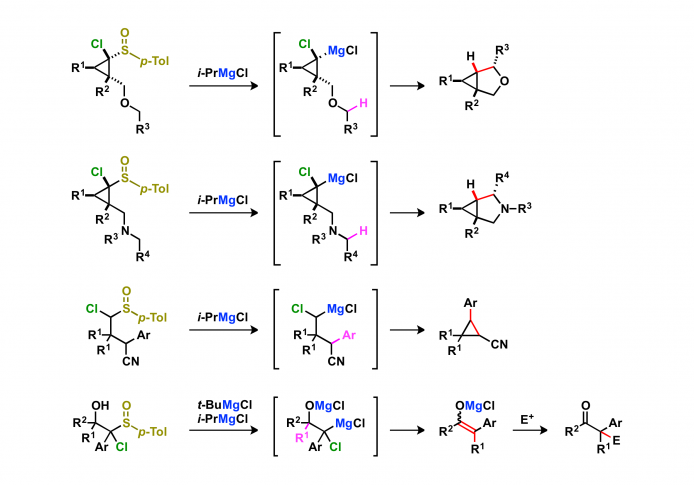
3. Carbene-like reactions
Intramolecular C–H and C–C insertions of magnesium carbenoids give cyclic compounds. The C–H and C–C insertions of magnesium carbenoids are useful method for the functionalization of unreactive bonds.
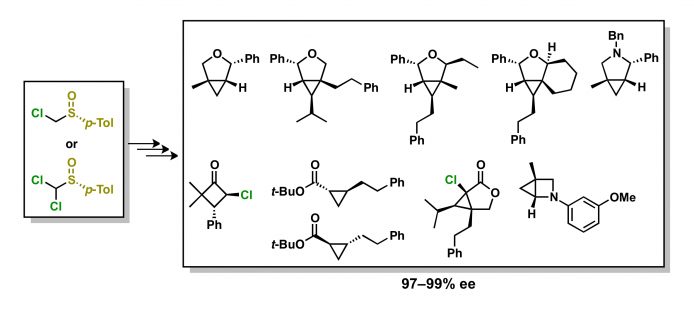
4. Asymmetric synthesis using a p-tolylsulfinyl group as a chiral auxiliary
The reaction of optically active α-chloro-substituted sulfoxides with Grignard reagents affords the products with high enantiomeric excess.
5. DFT study on the reaction mechanism of magnesium carbenoids
DFT calculations of magnesium carbenoids provide important clues on the unconventional reactivity of magnesium carbenoids. The degree of vinylidene character of α-heteroatom-substituted vinylmetals depends on the types of metal and α-heteroatom substituent. The activation free energies for the nucleophilic substitution of magnesium alkylidene carbenoid and cyclopropylmagnesium carbenoid with a chloride ion are lower than those of chloroethene and chlorocyclopropane. The disadvantages of the nucleophilic substitution of alkenyl halides and cyclopropyl halides seem to be relieved in the reaction of magnesium carbenoids due to their distorted structures. The 1,3-C–H insertion of magnesium carbenoid occurs according to an SN2-like mechanism wherein the C–H bond attacks the electrophilic carbenoid carbon atom.
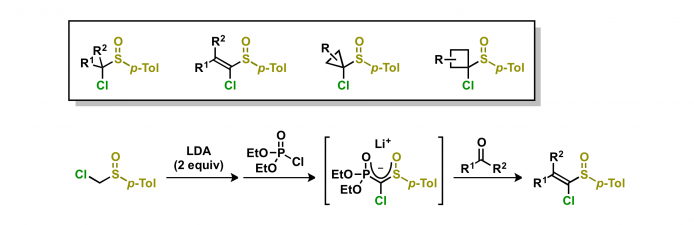
6. Development of efficient synthetic method for α-chloro-substituted sulfoxides
A conventional synthetic method for 1-chlorovinyl p-tolyl sulfoxides consists of three steps: (1) nucleophilic addition of [chloro(p-tolylsulfinyl)methyl]lithium to carbonyl compounds, (2) conversion of the hydroxy group of adducts to a leaving group, and (3) β-elimination. The Horner–Wadsworth–Emmons reaction of deprotonated phosphonate with carbonyl compounds gives 1-chlorovinyl p-tolyl sulfoxides in one-pot with high efficiency.
7. Conjugate addition of nucleophiles to 1-chlorovinyl p-tolyl sulfoxides
1-Chlorovinyl p-tolyl sulfoxides act as Michael acceptors toward the soft nucleophiles. The conjugate addition of lithium ester enolates to sulfoxides occurs in highly diastereoselective manner. Several kinds of nucleophiles consecutively reacted with 1-chlorovinyl p-tolyl sulfoxides to give products that consist of sulfoxide and nucleophile components in a ratio of 1:2.